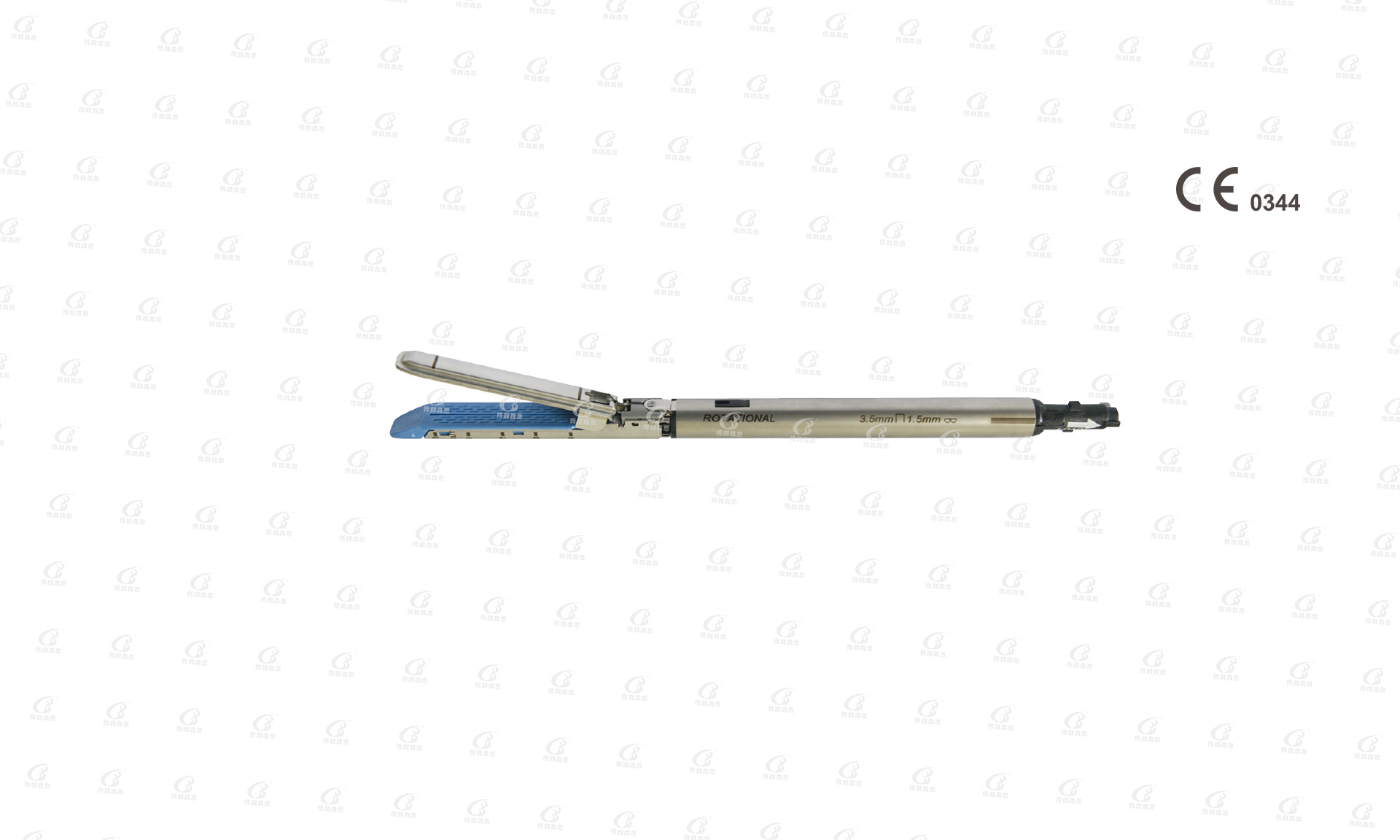
Disposable endocutter has three length types (shaft+handle), A type (standard endocutter)=370mm, B type (short endocutter)=320mm, L type (long endocutter)=470mm.

Reload model | Reload colour | Staple line length (mm) | Open staple height (mm) | Closed Staple Height(mm) |
JQZ-30-2.0/JQD-30-2.0 | Grey | 30 | 2.0 | 0.75 |
JQZ-40-2.0/JQD-45-2.0 | White | 30 | 2.5 | 1.0 |
JQZ-30-2.0/JQD-45-2.0 | Grey | 43 | 2.0 | 0.75 |
JQZ-45-2.5/JQD-45-2.5 | White | 43 | 2.5 | 1.0 |
JQZ-45-3.5/JQD-45-3.5 | Blue | 43 | 3.5 | 1.5 |
JQZ-45-4.0/JQD-45-4.0 | Golden | 43 | 4.0 | 1.75 |
JQZ-45-4.8/JQD-45-4.8 | Green | 43 | 4.8 | 2.0 |
JQZ-60-2.5/JQD-60-2.5 | White | 60 | 2.5 | 1.0 |
JQZ-60-3.5/JQD-60-3.5 | Blue | 60 | 3.5 | 1.5 |
JQZ-60-4.0/JQD-60-4.0 | Golden | 60 | 4.0 | 1.75 |
JQZ-60-4.8/JQD-60-4.8 | Green | 60 | 4.8 | 2.0 |
*Reloads include straight type (Z) and articulating type (D).
• Scope of application: This device is intended for use in abdominal, thoracic, and gynaecologic surgery for resection, transection, and creation of anastomosis. It simultaneously places two triple rows of staggered staples and cuts tissue between the two rows, completing the tissue anastomosis. Examples of procedures include: gastrectomy, appendectomy, pulmonary wedge resection, etc.
• Contraindications: Do not use where surgical stapling is not appropriate; do not use the short shaft JQ-B staplers for laparoscopic procedures, they are designed for open procedures only; do not not use the device where adequacy of haemostasis cannot be visually verified after application; do not use the device on the aorta and major blood vessels; do not use the device on ischemic, oedematous, friable, or necrotic tissue, as this could result in staple line leak or bleeding; do not use the reloads on tissue whose thickness cannot be comfortably compressed to less than the maximum value, or can be easily compressed to less than the minimum value of the corresponding tissue thickness range listed in the instructions for use, failure to respect the appropriate tissue thickness may lead to tissue damage or anastomosis leakage; do not use the device if the sterile barrier is damaged or unintentionally opened before use.